Simple analysis of the four major problems in the negative electrode of lithium battery
Editor's note
The output voltage of a lithium-ion battery is equal to the difference between the positive voltage and the negative voltage. Therefore, the lithium-ion voltage of the negative electrode of the lithium-ion battery determines the output voltage of the battery (at least half). The lower the operating voltage of the negative electrode, the operating voltage of the battery. The higher.
1. Where is the negative pole important?
What is the importance of the negative electrode of lithium-ion batteries? The output voltage of a lithium-ion battery is equal to the difference between the positive voltage and the negative voltage. Therefore, the lithium-ion voltage of the negative electrode of the lithium-ion battery determines the output voltage of the battery (at least half). The lower the operating voltage of the negative electrode, the operating voltage of the battery. The higher.
The specific capacity of the battery is determined by the combination of the positive specific capacity and the negative specific capacity, and the negative specific capacity determines at least half of the specific capacity of the battery.
The actual usable capacity of the battery is also related to the degree of tilt of the negative de-lithium voltage platform. The flatter the de-lithium voltage platform, the higher the capacity of the negative electrode, and the higher the specific capacity of the battery.
If the positive electrode capacity is P mAh/g and the negative electrode capacity is Q mAh/g, the theoretical specific capacity x of the battery satisfies the following formula.
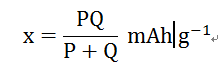
2. Quality is a compromise between energy and voltage
Strictly speaking, this compromise does not include lithium metal. The working voltage of lithium metal is 0V, the theoretical capacity is 3862mAh/g, and the actual capacity is determined by the utilization rate of active materials.
Why compromise? Generally speaking, the current negative electrode candidate material (excluding metallic lithium) has a basic feature that the larger the capacity of the active electrode material, the higher the delithiation voltage platform (or average value), for example, the average delithiation potential of the graphite-based carbon material is 0.15V, the actual capacity is 350mAh/g; the average de-lithium potential of the Sn anode is 0.5V, the theoretical capacity is 990mAh/g; the average de-lithium potential of the Si anode is 0.45V, and the theoretical capacity is 4200mAh/g. The actual usable capacity of Sn and Si is not yet determined and is ultimately determined by the conditions of use.
The energy density and specificity of the battery are determined by the product of the average operating voltage (positive and negative voltage difference) and the mass specific capacity (or volume specific capacity). When the carbon-based material is replaced by Si or Sn, can the capacity increase of the battery be compensated? The reduction in battery operating voltage is an important factor to consider. A simple example shows that if the positive electrode is lithium iron phosphate, its working voltage is 3.45V and the capacity is 160mAh/g; when it is matched with graphite carbon (measured by 0.15V, 350mAh/g actual capacity), 1g iron phosphate Lithium is matched with 0.457 g of graphitic carbon, and the theoretical specific energy of the whole cell is (3.45-0.15) V*160 mAh/1.457 g=362.3 mWh/g.
1g of lithium iron phosphate is matched with the negative electrode of Si, and the negative electrode of Si is calculated according to 0.45V and 4200mAh/g, and the specific energy of the matched whole battery is (3.45-0.45) V*160mAh/1.038g=462mWh/g. Obviously, after replacing the graphite carbon negative electrode with a Si negative electrode, its capacity increase can make up for the impact of the battery voltage drop caused by the increase in the delithiation voltage. Of course, this is a theoretical consideration because the actual capacity of the Si negative electrode cannot reach its theoretical value. Assuming that the actual capacity of the Si negative electrode is p, the condition that the total cell specific energy is greater than 362.3 mWh/g is satisfied, and the satisfied relationship is
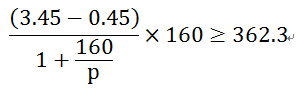
Of course, the above formula is still a little simplified, and the relationship between the voltage and capacity of the Si negative electrode is not considered, but it can explain the purpose we are currently discussing. The calculation yields p at least 492.5 mAh/g. In other words, the actual capacity of the Si negative electrode is greater than 492.5 mAh/g to ensure that the mass-to-cell characteristics of the whole cell are not deteriorated (compared with the lithium iron phosphate/graphite carbon system). The development of high-capacity C-Si composite anode materials can also be borrowed from the above discussion. Roughly speaking, the capacity of the Si portion of the C-Si composite anode in practice should not be less than 492.5 mAh/g, otherwise there is no significance.
This is the compromise between the two parameters of the negative de-lithium voltage increase and the reversible capacity increase. Because of the compromise between these two parameters, lithium titanate and nitride are directly ignored here, because the voltage platforms of the two are really high and cannot be tolerated.
3. High lithium state in composite anode materials
At present, it seems that the possibility of completely replacing graphite carbon anodes with Si, Sn, etc. alone is not large; the compromise scheme is the use of Si/C or Sn/C composite anodes, and the generalized is M/C composite anodes.
One of the biggest problems with M/C composite anodes is the presence of a high lithiated state. The high lithiation state is inevitable because the average delithiation voltage of M is higher than that of graphitic carbon. The high lithiation state is for M, and the high lithium state of M leads to some unexpected problems. The newly prepared M/C composite product is in a lithium-free state, from a lithium-free state to a high-lithiation state of M, accompanied by a drastic change in volume, and whether the initial microscopic relationship between M and C is maintained after lithiation. It is very important for cycle stability; in addition, there is a problem that the first charge and discharge coulombic efficiency is very low. Maybe this can be solved by pre-cycle, but the pre-circulation measures will be accompanied by the development of new battery manufacturing processes.
4. Negative research error
For lithium-ion batteries, the voltage cannot be operated to 0V; the specific characteristics are determined by the positive and negative voltage differences and the positive and negative pole capacities; this intrinsically determines that some voltage platforms are not obvious, or even obvious but higher than 1.0. V even has to work from 3.0V to 0.0V oxide, which has no practical application. Therefore, some studies on the behavior of deintercalation of lithium with oxides of the above characteristics have no meaning. For example, some studies on the deintercalation of lithium in materials such as copper oxide and iron oxide have no meaning.
AVS, AVSS, TXL, GXL, UL2464,UL1015,UL1007 normally used for car alarm wiring. JST, Dephi, Molex, and Chinese equipvalent part canbe assembly in the harness. Yacenter expands oversea markets, such as Europe, America,Janpan, etc. Owing to the rapid development, Yacenter has been awarded as [UL, CE, TS"certificate.
Any technical question, inquiry price, feel free to communicate with us.
Car Alarm Wiring,Car Alarm Wire Harness,Automobile Car Alarm Wire Harness,Car Stereo Alarm Wiring Harness
Dongguan YAC Electric Co,. LTD. , https://www.yacentercn.com